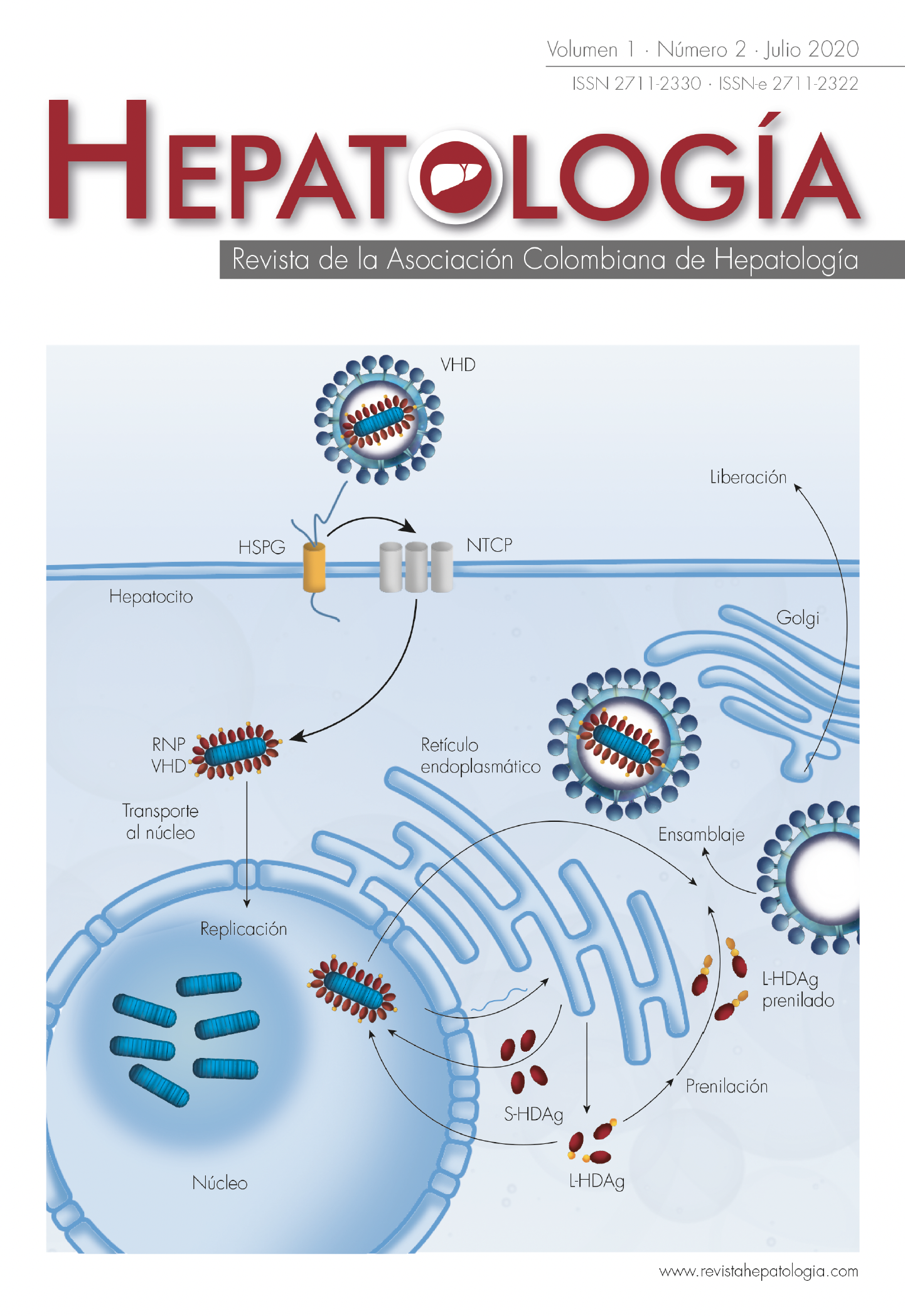
Infección por el virus de la hepatitis delta
DOI:
https://doi.org/10.52784/27112330.120Palabras clave:
virus de la hepatitis delta, virus de la hepatitis B, epidemiología, diagnóstico, tratamiento farmacológico.Resumen
El virus de la hepatitis delta (VHD) es un virus satélite del virus de la hepatitis B (VHB), dado que requiere el antígeno de superficie del VHB (HBsAg) para la producción de partículas virales infecciosas. Se han caracterizado ocho genotipos del VHD, con una distribución geográfica relacionada con la prevalencia de la infección por VHB. Se estima que aproximadamente el 5% de los pacientes con infección crónica por VHB también están infectados con VHD. Se han descrito dos tipos de infección: la coinfección simultánea por VHB y VHD, y la superinfección con VHD en un paciente previamente infectado por VHB, esta última asociada a una mayor morbilidad y mortalidad por falla hepática aguda. La infección se diagnostica en nuestro medio con la determinación de IgM contra el VHD, acompañada idealmente de la carga viral. Aunque el tratamiento de elección es la terapia con interferón alfa pegilado, en el momento se están evaluando otros medicamentos antivirales en ensayos clínicos, con resultados alentadores, teniendo en cuenta el efecto observado en la carga viral del VHD y/o del VHB en los pacientes. La presente revisión tiene como objetivo incluir temas como la biología del virus, la epidemiología, las características clínicas, el diagnóstico y el tratamiento en la infección por VHD.
Descargas
Referencias bibliográficas
Botelho-Souza LF, Pinheiro-Alves Vasconcelos M, de Oliveira-Dos Santos A, Villalobos-Salcedo JM, Souza-Vieira D. Hepatitis delta: virological and clinical aspects. Virol J 2017;14:177. https://doi.org/10.1186/s12985-017-0845-y.
Fu TB, Taylor J. The RNAs of hepatitis delta virus are copied by RNA polymerase II in nuclear homogenates. J Virol 1993;67:6965-6972.
Mentha N, Clement S, Negro F, Alfaiate D. A review on hepatitis D: From virology to new therapies. J Adv Res 2019;17:3-15. https://doi.org/10.1016/j.jare.2019.03.009.
World Health Organization (WHO). Hepatitis D (Fact sheets). Ginebra, Suiza: World Health Organization; 2019. Acceso 19 de julio de 2019. Disponible en https://www.who.int/news-room/fact-sheets/detail/hepatitis-d.
Rizzetto M. The adventure of delta. Liver Int 2016;36 Suppl 1:135-140. https://doi.org/10.1111/liv.13018.
Wang TC, Chao M. Molecular cloning and expression of the hepatitis delta virus genotype IIb genome. Biochem Biophys Res Commun 2003;303:357-363. https://doi.org/10.1016/s0006-291x(03)00338-3.
Chao YC, Chang MF, Gust I, Lai MM. Sequence conservation and divergence of hepatitis delta virus RNA. Virology 1990;178:384-392. https://doi.org/10.1016/0042-6822(90)90335-o.
Li J, Tong S. From DCPD to NTCP: the long journey towards identifying a functional hepatitis B virus receptor. Clin Mol Hepatol 2015;21:193-199. https://doi.org/10.3350/cmh.2015.21.3.193.
Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012;1:e00049. https://doi.org/10.7554/eLife.00049.
Alves C, Freitas N, Cunha C. Characterization of the nuclear localization signal of the hepatitis delta virus antigen. Virology 2008;370:12-21. https://doi.org/10.1016/j.virol.2007.07.034.
Chou HC, Hsieh TY, Sheu GT, Lai MM. Hepatitis delta antigen mediates the nuclear import of hepatitis delta virus RNA. J Virol 1998;72:3684-3690.
Flores R, Grubb D, Elleuch A, Nohales MA, Delgado S, Gago S. Rolling-circle replication of viroids, viroid-like satellite RNAs and hepatitis delta virus: variations on a theme. RNA Biol 2011;8:200-206. https://doi.org/10.4161/rna.8.2.14238.
Modahl LE, Macnaughton TB, Zhu N, Johnson DL, Lai MM. RNA-dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol Cell Biol 2000;20:6030-6039. https://doi.org/10.1128/mcb.20.16.6030-6039.2000.
Chang J, Nie X, Chang HE, Han Z, Taylor J. Transcription of hepatitis delta virus RNA by RNA polymerase II. J Virol 2008;82:1118-1127. https://doi.org/10.1128/JVI.01758-07.
Casey JL. Control of ADAR1 editing of hepatitis delta virus RNAs. Curr Top Microbiol Immunol 2012;353:123-143. https://doi.org/10.1007/82_2011_146.
Abbas Z, Afzal R. Life cycle and pathogenesis of hepatitis D virus: A review. World J Hepatol 2013;5:666-675. https://doi.org/10.4254/wjh.v5.i12.666.
Hong SY, Chen PJ. Phosphorylation of serine 177 of the small hepatitis delta antigen regulates viral antigenomic RNA replication by interacting with the processive RNA polymerase II. J Virol 2010;84:1430-1438. https://doi.org/10.1128/JVI.02083-09.
Li YJ, Stallcup MR, Lai MM. Hepatitis delta virus antigen is methylated at arginine residues, and methylation regulates subcellular localization and RNA replication. J Virol 2004;78:13325-13334. https://doi.org/10.1128/JVI.78.23.13325-13334.2004.
Tseng CH, Cheng TS, Shu CY, Jeng KS, Lai MM. Modification of small hepatitis delta virus antigen by SUMO protein. J Virol 2010;84:918-927. https://doi.org/10.1128/JVI.01034-09.
Greco-Stewart V, Pelchat M. Interaction of host cellular proteins with components of the hepatitis delta virus. Viruses 2010;2:189-212. https://doi.org/10.3390/v2010189.
Tavanez JP, Cunha C, Silva MC, David E, Monjardino J, Carmo-Fonseca M. Hepatitis delta virus ribonucleoproteins shuttle between the nucleus and the cytoplasm. RNA 2002;8:637-646. https://doi.org/10.1017/s1355838202026432.
Sureau C, Negro F. The hepatitis delta virus: Replication and pathogenesis. J Hepatol 2016;64:S102-S116. https://doi.org/10.1016/j.jhep.2016.02.013.
Perez-Vargas J, Amirache F, Boson B, Mialon C, Freitas N, Sureau C, et al. Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo. Nat Commun 2019;10:2098. https://doi.org/10.1038/s41467-019-10117-z.
Chen HY, Shen DT, Ji DZ, Han PC, Zhang WM, Ma JF, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut 2018;68:512-521. https://doi.org/10.1136/gutjnl-2018-316601.
Botelho-Souza LF, Souza Vieira D, de Oliveira Dos Santos A, Cunha Pereira AV, Villalobos-Salcedo JM. Characterization of the genotypic profile of hepatitis delta virus: Isolation of HDV genotype-1 in the Western Amazon region of Brazil. Intervirology 2015;58:166-171. https://doi.org/10.1159/000431040.
Le Gal F, Gault E, Ripault MP, Serpaggi J, Trinchet JC, Gordien E, et al. Eighth major clade for hepatitis delta virus. Emerg Infect Dis 2006;12:1447-1450. https://doi.org/10.3201/eid1209.060112.
Shakil AO, Hadziyannis S, Hoofnagle JH, Di Bisceglie AM, Gerin JL, Casey JL. Geographic distribution and genetic variability of hepatitis delta virus genotype I. Virology 1997;234:160-167. https://doi.org/10.1006/viro.1997.8644.
Casey JL, Brown TL, Colan EJ, Wignall FS, Gerin JL. A genotype of hepatitis D virus that occurs in northern South America. Proc Natl Acad Sci USA 1993;90:9016-9020. https://doi.org/10.1073/pnas.90.19.9016.
Gilman C, Heller T, Koh C. Chronic hepatitis delta: A state-of-the-art review and new therapies. World J Gastroenterol 2019;25:4580-4597. https://doi.org/10.3748/wjg.v25.i32.4580.
Alvarado-Mora MV, Romano CM, Gomes-Gouvea MS, Gutierrez MF, Carrilho FJ, Pinho JR. Dynamics of hepatitis D (delta) virus genotype 3 in the Amazon region of South America. Infect Genet Evol 2011;11:1462-1468. https://doi.org/10.1016/j.meegid.2011.05.020.
Radjef N, Gordien E, Ivaniushina V, Gault E, Anais P, Drugan T, et al. Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. J Virol 2004;78:2537-2544. https://doi.org/10.1128/jvi.78.5.2537-2544.2004.
Barros LM, Gomes-Gouvea MS, Pinho JR, Alvarado-Mora MV, Dos Santos A, Mendes-Correa MC, et al. Hepatitis delta virus genotype 8 infection in Northeast Brazil: inheritance from African slaves? Virus Res 2011;160:333-339. https://doi.org/10.1016/j.virusres.2011.07.006.
Torres JR. Hepatitis B and hepatitis delta virus infection in South America. Gut 1996;38 Suppl 2:S48-S55. https://doi.org/10.1136/gut.38.suppl_2.s48.
Crispim MA, Fraiji NA, Campello SC, Schriefer NA, Stefani MM, Kiesslich D. Molecular epidemiology of hepatitis B and hepatitis delta viruses circulating in the Western Amazon region, North Brazil. BMC Infect Dis 2014;14:94. https://doi.org/10.1186/1471-2334-14-94.
Gomes-Gouvea MS, Soares MCP, Bensabath G, de Carvalho-Mello I, Brito EMF, Souza OSC, et al. Hepatitis B virus and hepatitis delta virus genotypes in outbreaks of fulminant hepatitis (Labrea black fever) in the Western Brazilian Amazon region. J Gen Virol 2009;90:2638-2643. https://doi.org/10.1099/vir.0.013615-0.
Dias LB, Moraes MA. [Labrea hepatitis]. Rev Inst Med Trop Sao Paulo 1973;15:86-93.
Aguilera A, Morales A, Buitrago B, Guzmán M, Peña C, Marquez G. Hepatitis fulminante epidémica de la Sierra Nevada de Santa Marta I. Estudio de un brote en la localidad de Julio Zawady, Ciénaga, Magdalena Colombia. Biomedica 1969;1:187-197. https://doi.org/10.7705/biomedica.v1i4.1801.
Padilla JC, Arriaga AL. Hepatitis A, B y D en Chocó. Biomedica 1997;17:286-291. https://doi.org/10.7705/biomedica.v17i4.959.
Torres JR, Mondolfi A. Protracted outbreak of severe delta hepatitis: experience in an isolated Amerindian population of the Upper Orinoco basin. Rev Infect Dis 1991;13:52-55. https://doi.org/10.1093/clinids/13.1.52.
di Filippo Villa D, Cortes-Mancera F, Payares E, Montes N, de la Hoz F, Arbelaez MP, et al. Hepatitis D virus and hepatitis B virus infection in Amerindian communities of the Amazonas state, Colombia. Virol J 2015;12:172. https://doi.org/10.1186/s12985-015-0402-5.
Braga WS. [Hepatitis B and D virus infection within Amerindians ethnic groups in the Brazilian Amazon: epidemiological aspects]. Rev Soc Bras Med Trop 2004;37 Suppl 2:9-13. https://doi.org/10.1590/s0037-86822004000700002.
Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008;48:335-352. https://doi.org/10.1016/j.jhep.2007.11.011.
Niro GA, Casey JL, Gravinese E, Garrubba M, Conoscitore P, Sagnelli E, et al. Intrafamilial transmission of hepatitis delta virus: molecular evidence. J Hepatol 1999;30:564-569. https://doi.org/10.1016/s0168-8278(99)80185-8.
Ramia S, Bahakim H. Perinatal transmission of hepatitis B virus-associated hepatitis D virus. Ann Inst Pasteur Virol 1988;139:285-290. https://doi.org/10.1016/s0769-2617(88)80041-8.
Le Gal F, Brichler S, Drugan T, Alloui C, Roulot D, Pawlotsky JM, et al. Genetic diversity and worldwide distribution of the deltavirus genus: A study of 2,152 clinical strains. Hepatology 2017;66:1826-1841. https://doi.org/10.1002/hep.29574.
Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet 2011;378:73-85. https://doi.org/10.1016/S0140-6736(10)61931-9.
Parana R, Pujol FH. Clinical and virological heterogeneity of hepatitis delta in the Amazonia: More questions than answers. Clin Liver Dis (Hoboken) 2019;13:62-65. https://doi.org/10.1002/cld.794.
Shih HH, Jeng KS, Syu WJ, Huang YH, Su CW, Peng WL, et al. Hepatitis B surface antigen levels and sequences of natural hepatitis B virus variants influence the assembly and secretion of hepatitis D virus. J Virol 2008;82:2250-2264. https://doi.org/10.1128/JVI.02155-07.
Braga WS, de Oliveira CM, de Araujo JR, Castilho Mda C, Rocha JM, Gimaque JB, et al. Chronic HDV/HBV co-infection: predictors of disease stage: a case series of HDV-3 patients. J Hepatol 2014;61:1205-1211. https://doi.org/10.1016/j.jhep.2014.05.041.
Guilhot S, Huang SN, Xia YP, La Monica N, Lai MM, Chisari FV. Expression of the hepatitis delta virus large and small antigens in transgenic mice. J Virol 1994;68:1052-1058.
Negro F, Baldi M, Bonino F, Rocca G, Demartini A, Passarino G, et al. Chronic HDV (hepatitis delta virus) hepatitis. Intrahepatic expression of delta antigen, histologic activity and outcome of liver disease. J Hepatol 1988;6:8-14. https://doi.org/10.1016/s0168-8278(88)80457-4.
Giersch K, Allweiss L, Volz T, Helbig M, Bierwolf J, Lohse AW, et al. Hepatitis delta co-infection in humanized mice leads to pronounced induction of innate immune responses in comparison to HBV mono-infection. J Hepatol 2015;63:346-353. https://doi.org/10.1016/j.jhep.2015.03.011.
Williams V, Brichler S, Radjef N, Lebon P, Goffard A, Hober D, et al. Hepatitis delta virus proteins repress hepatitis B virus enhancers and activate the alpha/beta interferon-inducible MxA gene. J Gen Virol 2009;90:2759-2767. https://doi.org/10.1099/vir.0.011239-0.
Lunemann S, Malone DF, Grabowski J, Port K, Beziat V, Bremer B, et al. Effects of HDV infection and pegylated interferon alpha treatment on the natural killer cell compartment in chronically infected individuals. Gut 2015;64:469-482. https://doi.org/10.1136/gutjnl-2014-306767.
Lunemann S, Malone DF, Hengst J, Port K, Grabowski J, Deterding K, et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis 2014;209:1362-1373. https://doi.org/10.1093/infdis/jit561.
Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J Gastroenterol 2019;25:3527-3537. https://doi.org/10.3748/wjg.v25.i27.3527.
Aslan N, Yurdaydin C, Bozkaya H, Baglan P, Bozdayi AM, Tillmann HL, et al. Analysis and function of delta-hepatitis virus-specific cellular immune responses. J Hepatol 2003;38:15-16. https://doi.org/10.1016/S0168-8278(03)80457-9.
Wedemeyer H, Ciner A, Yurdaydìn C, Zachou K, Heidrich B, Manns M. Differential cytokine pattern of HDV-specific cellular immune responses: Results from the hep-net/international HIDIT-1 study. Z Gastroenterol 2007;45:V05. https://doi.org/10.1055/s-2007-988123.
Huang YH, Tao MH, Hu CP, Syu WJ, Wu JC. Identification of novel HLA-A*0201-restricted CD8+ T-cell epitopes on hepatitis delta virus. J Gen Virol 2004;85:3089-3098. https://doi.org/10.1099/vir.0.80183-0.
Smedile A, Farci P, Verme G, Caredda F, Cargnel A, Caporaso N, et al. Influence of delta infection on severity of hepatitis B. Lancet 1982;2:945-947. https://doi.org/10.1016/s0140-6736(82)90156-8.
Taylor JM. Structure and replication of hepatitis delta virus RNA. Curr Top Microbiol Immunol 2006;307:1-23. https://doi.org/10.1007/3-540-29802-9_1.
Farci P, Niro GA. Clinical features of hepatitis D. Semin Liver Dis 2012;32:228-236. https://doi.org/10.1055/s-0032-1323628.
Rizzetto M, Durazzo M. Hepatitis delta virus (HDV) infections. Epidemiological and clinical heterogeneity. J Hepatol 1991;13 Suppl 4:S116-118. https://doi.org/10.1016/0168-8278(91)90040-i.
Romeo R, Del Ninno E, Rumi M, Russo A, Sangiovanni A, de Franchis R, et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 2009;136:1629-1638. https://doi.org/10.1053/j.gastro.2009.01.052.
Negro F. Hepatitis D virus coinfection and superinfection. Cold Spring Harb Perspect Med 2014;4:a021550. https://doi.org/10.1101/cshperspect.a021550.
Pascarella S, Negro F. Hepatitis D virus: an update. Liver Int 2011;31:7-21. https://doi.org/10.1111/j.1478-3231.2010.02320.x.
Yurdaydin C, Idilman R, Bozkaya H, Bozdayi AM. Natural history and treatment of chronic delta hepatitis. J Viral Hepat 2010;17:749-756. https://doi.org/10.1111/j.1365-2893.2010.01353.x.
Rizzetto M. Hepatitis D: virology, clinical and epidemiological aspects. Acta Gastroenterol Belg 2000;63:221-224.
Sagnelli E, Felaco FM, Filippini P, Pasquale G, Peinetti P, Buonagurio E, et al. Influence of HDV infection on clinical, biochemical and histological presentation of HBsAg positive chronic hepatitis. Liver 1989;9:229-234. https://doi.org/10.1111/j.1600-0676.1989.tb00404.x.
Fattovich G, Boscaro S, Noventa F, Pornaro E, Stenico D, Alberti A, et al. Influence of hepatitis delta virus infection on progression to cirrhosis in chronic hepatitis type B. J Infect Dis 1987;155:931-935. https://doi.org/10.1093/infdis/155.5.931.
Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut 2000;46:420-426. https://doi.org/10.1136/gut.46.3.420.
Sanchez-Tapias JM, Mas A, Costa J, Bruguera M, Mayor A, Ballesta AM, et al. Recombinant alpha 2c-interferon therapy in fulminant viral hepatitis. J Hepatol 1987;5:205-210. https://doi.org/10.1016/s0168-8278(87)80574-3.
World Health Organization (WHO). Hepatitis B. Ginebra, Suiza: World Health Organization; 2019. Acceso 19 de julio de 2019. Disponible en https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b.
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-398. https://doi.org/10.1016/j.jhep.2017.03.021.
Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: Prevalence, risk factors, and outcomes. J Hepatol 2015;63:586-592. https://doi.org/10.1016/j.jhep.2015.04.025.
Patel EU, Thio CL, Boon D, Thomas DL, Tobian AAR. Prevalence of hepatitis B and Hepatitis D virus infections in the United States, 2011-2016. Clin Infect Dis 2019;69:709-712. https://doi.org/10.1093/cid/ciz001.
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560-1599. https://doi.org/10.1002/hep.29800.
Mederacke I, Bremer B, Heidrich B, Kirschner J, Deterding K, Bock T, et al. Establishment of a novel quantitative hepatitis D virus (HDV) RNA assay using the Cobas TaqMan platform to study HDV RNA kinetics. J Clin Microbiol 2010;48:2022-2029. https://doi.org/10.1128/JCM.00084-10.
Zachou K, Yurdaydin C, Drebber U, Dalekos GN, Erhardt A, Cakaloglu Y, et al. Quantitative HBsAg and HDV-RNA levels in chronic delta hepatitis. Liver Int 2010;30:430-437. https://doi.org/10.1111/j.1478-3231.2009.02140.x.
Manesis EK, Schina M, Le Gal F, Agelopoulou O, Papaioannou C, Kalligeros C, et al. Quantitative analysis of hepatitis D virus RNA and hepatitis B surface antigen serum levels in chronic delta hepatitis improves treatment monitoring. Antivir Ther 2007;12:381-388.
Le Gal F, Gordien E, Affolabi D, Hanslik T, Alloui C, Deny P, et al. Quantification of hepatitis delta virus RNA in serum by consensus real-time PCR indicates different patterns of virological response to interferon therapy in chronically infected patients. J Clin Microbiol 2005;43:2363-2369. https://doi.org/10.1128/JCM.43.5.2363-2369.2005.
Castelnau C, Le Gal F, Ripault MP, Gordien E, Martinot-Peignoux M, Boyer N, et al. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology 2006;44:728-735. https://doi.org/10.1002/hep.21325.
Le Gal F, Brichler S, Sahli R, Chevret S, Gordien E. First international external quality assessment for hepatitis delta virus RNA quantification in plasma. Hepatology 2016;64:1483-1494. https://doi.org/10.1002/hep.28772.
Le Gal F, Dziri S, Gerber A, Alloui C, Ben Abdesselam Z, Roulot D, et al. Performance characteristics of a new consensus commercial kit for hepatitis D virus RNA viral load quantification. J Clin Microbiol 2017;55:431-441. https://doi.org/10.1128/JCM.02027-16.
Ahn J, Gish RG. Hepatitis D virus: A call to screening. Gastroenterol Hepatol 2014;10:647-686.
Masood U, John S. Hepatitis D. StatPearls. Treasure Island (FL): StatPearls Publishing; 2019. Acceso 10 de enero de 2020. Disponible en https://www.ncbi.nlm.nih.gov/books/NBK470436/.
Niro GA, Rosina F, Rizzetto M. Treatment of hepatitis D. J Viral Hepat 2005;12:2-9. https://doi.org/10.1111/j.1365-2893.2005.00601.x.
Heidrich B, Yurdaydin C, Kabacam G, Ratsch BA, Zachou K, Bremer B, et al. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology 2014;60:87-97. https://doi.org/10.1002/hep.27102.
Yurdaydin C. New treatment options for delta virus: Is a cure in sight? J Viral Hepat 2019;26:618-626. https://doi.org/10.1111/jvh.13081.
Koh C, Canini L, Dahari H, Zhao X, Uprichard SL, Haynes-Williams V, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis 2015;15:1167-1174. https://doi.org/10.1016/S1473-3099(15)00074-2.
Yurdaydin C, Idilman R, Choong I, Kalkan C, Keskin O, Karakaya MF, et al. O118: Optimizing the prenylation inhibitor lonafarnib using ritonavir boosting in patients with chronic delta hepatitis. J Hepatol 2015;62:S252. https://doi.org/10.1016/S0168-8278(15)30137-9.
Yurdaydin C, Keskin O, Kalkan Ç, Karakaya F, Çalişkan A, Karatayli E, et al. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The LOWR HDV-1 study. Hepatology 2018;67:1224-1236. https://doi.org/10.1002/hep.29658.
National Cancer Institute. NCI Drug Dictionary. Lonafarnib. Bethesta, Maryland: NCI. Acceso 20 de enero de 2020. Disponible en https://www.cancer.gov/publications/dictionaries/cancer-drug/def/lonafarnib.
Bazinet M, Pantea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2017;2:877-889. https://doi.org/10.1016/S2468-1253(17)30288-1.
Shekhtman L, Cotler SJ, Hershkovich L, Uprichard SL, Bazinet M, Pantea V, et al. Modelling hepatitis D virus RNA and HBsAg dynamics during nucleic acid polymer monotherapy suggest rapid turnover of HBsAg. Sci Rep 2020;10:7837. https://doi.org/10.1038/s41598-020-64122-0.
Caviglia GP, Rizzetto M. Treatment of hepatitis D: an unmet medical need. Clin Microbiol Infect 2020;26:824-827. https://doi.org/10.1016/j.cmi.2020.02.031.
Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol 2016;65:490-498. https://doi.org/10.1016/j.jhep.2016.04.016.
Blank A, Markert C, Hohmann N, Carls A, Mikus G, Lehr T, et al. First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J Hepatol 2016;65:483-489. https://doi.org/10.1016/j.jhep.2016.04.013.
Yurdaydin C. Recent advances in managing hepatitis D. F1000Res 2017;6:1596. https://doi.org/10.12688/f1000research.11796.1.
Descargas
Publicado
Cómo citar
Número
Sección
Licencia

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
La política de reconocimiento de autoría de Hepatología se basa en los criterios indicados por el ICMJE para conferir crédito de un trabajo a una persona, a la vez que se le atribuye responsabilidad por la publicación del mismo.